Another person has died in an outbreak connected to contaminated eye drops, and more people are experiencing vision loss. According to an update given by the Centers for Disease Control and Prevention on Friday and first reported by ABC News, the death toll has grown to four. One of the deaths happened in Washington state, but the CDC provided no details on the other victims.
Furthermore, at least 14 persons have gone blind, up from eight recorded in the previous update in March. Four people had their eyeballs surgically removed, but the figure has not increased.
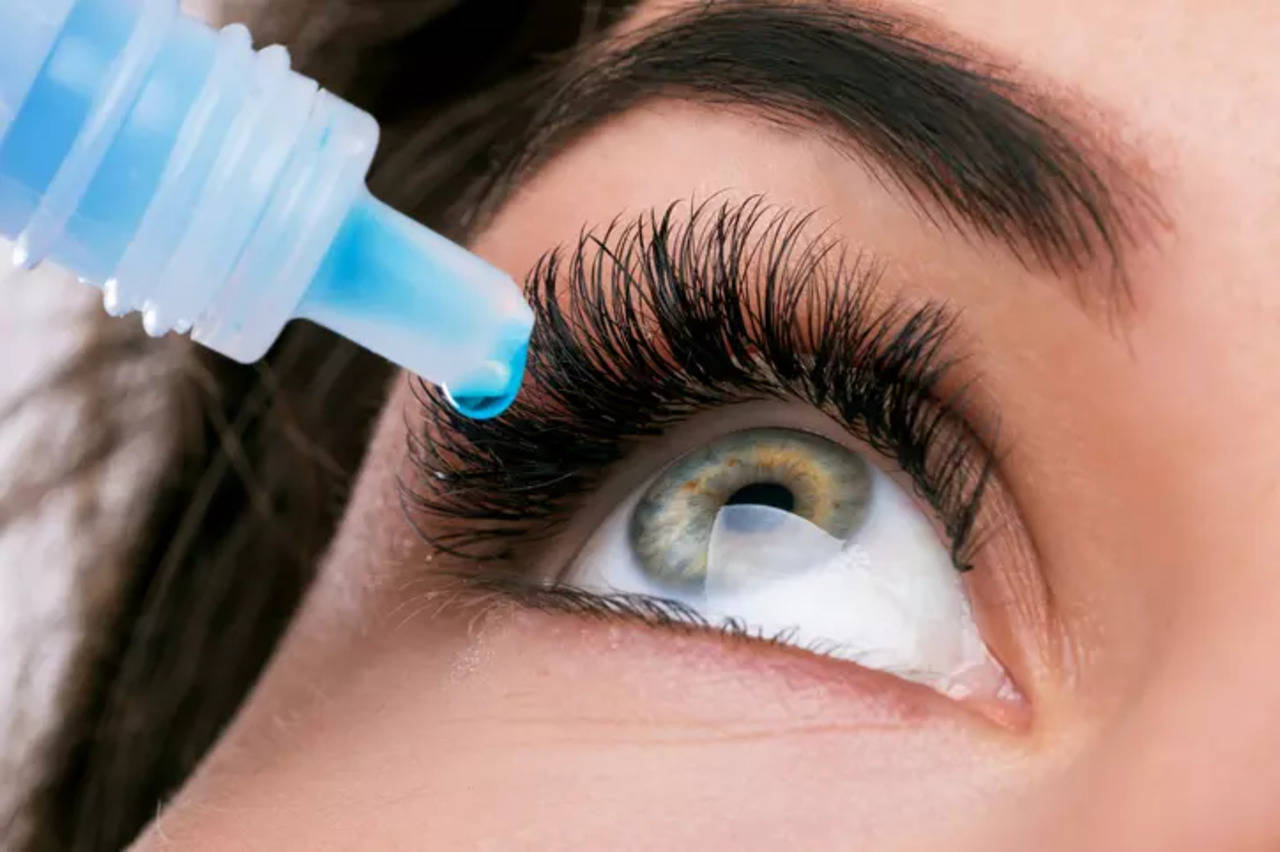
Patients claimed to have used at least ten different brands of fake tears, although the majority of cases have been attributed to EzriCare and Delsam Pharma eye drops, made by India-based Global Pharma Healthcare.
The strain connected to the outbreak has never been recorded in the United States before
According to the CDC, the eye drops were tainted with an antibiotic-resistant strain of Pseudomonas aeruginosa, an aggressive bacterium.
Pseudomonas is a species of bacteria present in the environment, with P. aeruginosa being the most common cause of human diseases.
The infection is frequent in healthcare settings and spreads because of poor hygiene, such as dirty hands or medical equipment and surfaces that are not thoroughly cleansed.
According to the CDC, P. aeruginosa is resistant to various antibiotics and has caused around 32,600 infections among hospitalized patients in the United States, as well as an estimated 2,700 deaths.
The strain connected to the outbreak, on the other hand, had never been recorded in the United States before, according to the CDC’s bulletin.
P. aeruginosa had infected 81 people across 18 states as of May 15, an increase of 13 patients since the previous update.
Yellow, green, or clear discharge from the eye; eye pain or discomfort; red eyes or eyelids; a feeling of something in the eye; increased sensitivity to light; and impaired vision are all symptoms of their infections.
Recall and Contamination of EzriCare and Delsam Pharma’s Artificial Tears: Continued Cases Reported
The FDA issued a warning in February, backed up by the CDC, asking physicians and the general public not to purchase EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears owing to probable bacterial contamination.
Following the warning, Global Pharma Healthcare initiated a voluntary recall of both products, alerting distributors and recommending wholesalers, retailers, and customers to discontinue use. Global Health Pharmaceutical has also issued a recall of Delsam Pharma’s Artificial Ointment.
The CDC and the FDA are advising anyone who still owns these brands to cease using them immediately and discard them. None of the products appear to be available for purchase online.
Six of the 13 new cases reported to the CDC had material taken before the February recall.
“These cases were confirmed after the recall date due to the time it takes for testing to confirm the outbreak strain and because of retrospective reporting of infections,” the CDC wrote in its update.
The seven patients whose specimens were collected after the recall was either in long-term care facilities with previous known cases or were using a recalled brand of artificial tears.






