The US FDA on Tuesday authorized the use of Pfizer’s omicron single-dose booster dose for children under the age of five. Read to know more.
Pfizer booster dose approved for kids under 5y
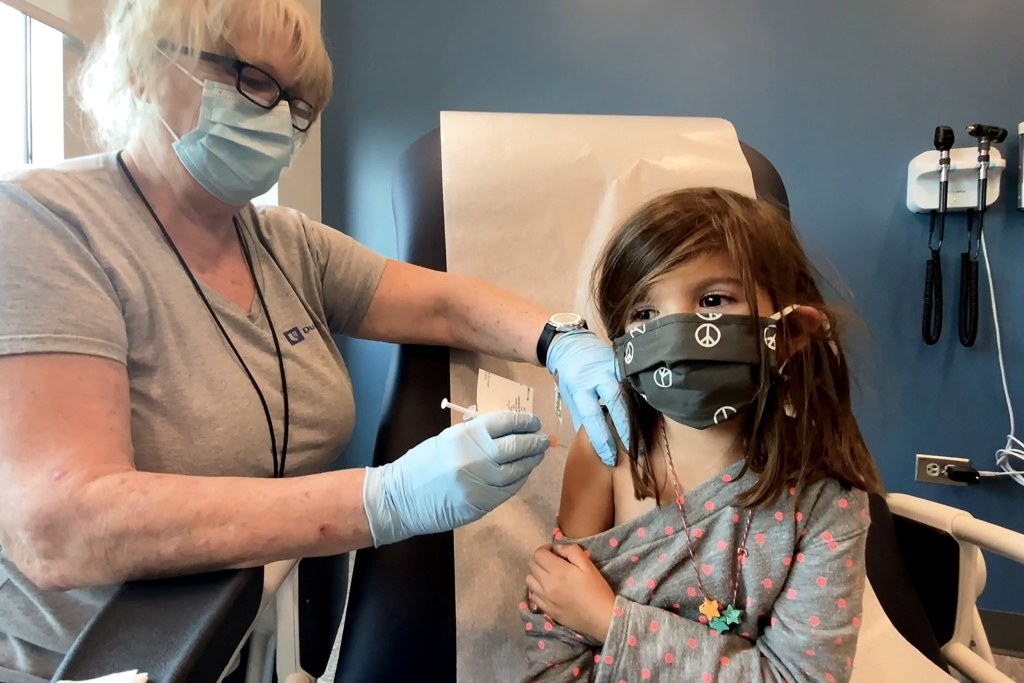
On Tuesday, the US Food and Drug Administration (FDA) authorized the Pfizer single-dose booster shot for children under the age of five. Children who were previously vaccinated with three doses of the vaccine can now receive the shot. As per the FDA, the emergency use authorization was following a clinical study. The report from the trial was based on 60 children belonging to the age group. One month after receiving the vaccination, the children’s immune systems displayed effective protection. This is against both the original and Omicron BA.4/BA.5 variant of the virus.
“Today’s authorization provides parents and caregivers of children 6 months through 4 years of age. Who received the three-dose primary series with the monovalent Pfizer-BioNTech COVID-19 Vaccine. An opportunity to update their children’s protection by receiving a booster dose with the Pfizer-BioNTech COVID-19 Vaccine, Bivalent,” stated Peter Marks. Marks is the director of the Center for Biologics Evaluation and Research under the FDA.
More on the update
As per data released by the US government, the uptake of the initial doses was abysmally slow in children. So far, only 2.7 percent of eligible children under the age of two received the vaccination. Similarly, for children under the age of five, it is five percent. Moreover, shots for the age group were approved in June 2022, making them the last to become eligible.
“Currently available data also show that vaccination remains the best defense against severe disease, hospitalization, and death caused by COVID-19 across all age groups. And we encourage all eligible individuals to make sure that their vaccinations are up to date with a bivalent COVID-19 vaccine,” added Marks.






