
President Biden tested positive for COVID-19 and is responding favorably to Paxlovid treatment. Here’s all you need to know about the antiviral covid drug treatment.
On Thursday, in a letter to the White House press secretary, Kevin C. O’Connor, the presidential physician confirmed President Biden tested positive for COVID-19. He is currently resting and experiencing symptoms such as fatigue and a runny nose. In the letter, Dr. O’Connor expresses Biden to “respond favorably to treatment” of Paxlovid.
What is Paxlovid?
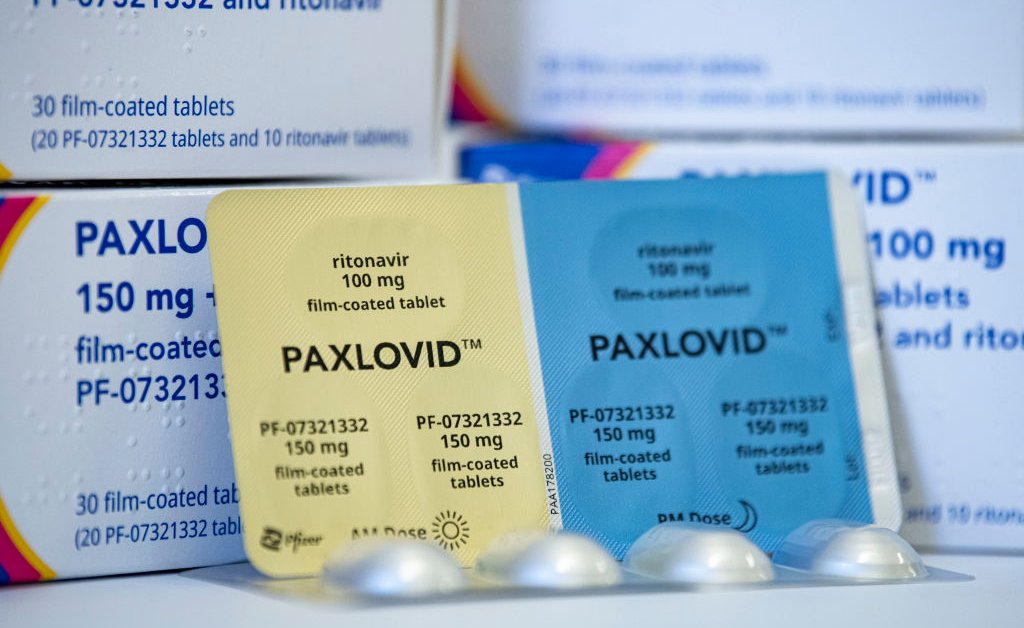
Paxlovid is an antiviral pill produced and developed by Pfizer. It is an oral pill aimed at limiting the severity of COVID-19. Approved by the US Centers for Disease Control & Prevention (CDC) in December 2021, it is focused on mild to moderate symptoms.
According to studies, it helps reduce the risk of death and hospitalization in highly vulnerable patients by 90 percent.
The antiviral pill prevents the virus proteins from multiplying in the body during the initial five days of infection. Hence allowing the immune system to fight it. It contains two active ingredients- the medication, PF-07321332, and ritonavir. The former is the antiviral medication. However, ritonavir is responsible for slowing down the breakdown of the antiviral medication. It has two tablets, ritonavir, and nirmatrelvir. It must be taken together, twice a day for five days.
More on the antiviral medication:
As per the CDC “People who are highly vulnerable to hospitalization and death from COVID-19 can access Paxlovid in the US”. This includes people over the age of 65 and those with underlying conditions such as diabetes, obesity, cancer, etc. However, those suffering from kidney disease must take a lower dose. To be eligible to take Paxlovid, patients must weigh 88 lbs or more and be older than 12 years. According to a Yale study, it is available through healthcare providers after testing positive for the virus. However, despite some restrictions, the drug is available from a state-licensed pharmacist.
Compared to Merck’s Lagevrio, Paxlovid’s closest rival, the antiviral drug is 60 percent more effective. Additionally, according to the Financial Times, Lagerviro has a 30 percent chance of preventing death or severe illness. A late-stage trial revealed that Paxlovid reduces hospitalization and death by 89 percent.
